Renewing the Molecular Transformation Process with Catalysts to Create Novel Molecules.
The origin of chemistry is "manufacturing". In addition to selective conversion of substances to produce only what is desired, organic chemistry requires the development of environmentally benign molecular transformation processes that reduce waste associated with material production.
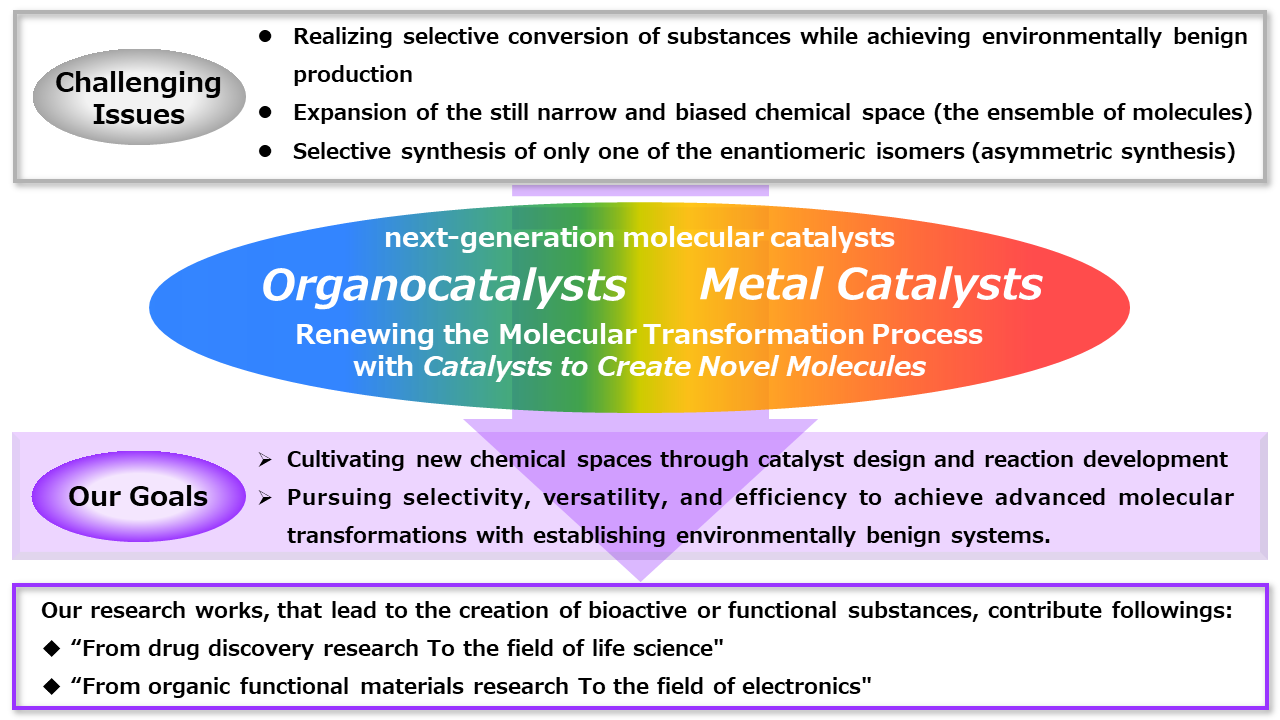
While chemists have developed many types of chemical reactions to synthesize molecules, it has been estimated that more than 1060 small molecules can be considered as candidate compounds, for example, pharmaceuticals or pesticides. However, the chemical space (the ensemble of molecules) of the actually synthesized molecules is still narrow and biased, and further expansion of the chemical space is required. Along with the creation of these new molecules, the selective synthesis of only one of the enantiomeric isomers (asymmetric synthesis) has become an indispensable methodology for the manufacture of biologically active molecules that exhibit medicinal properties, such as pharmaceuticals and pesticides. Realizing selective conversion of substances while achieving environmentally benign production is also one of the important issues required for the advanced molecular transformation processes.
In our laboratory, we pursue selectivity, versatility, and efficiency by creating next-generation molecular catalysts that take advantage of the characteristics of organic molecules and transition metal complexes and develop new molecular transformation processes that respond to the creation of the new ensemble of molecules. Based on catalyst design and reaction development, we aim to cultivate new chemical spaces, and to advance molecular transformations as well as to achieve environmentally benign molecular transformations. We are working on the following four main themes with the ultimate goal of contributing "from drug discovery research to the field of life science" and "from organic functional materials research to the field of electronics" through research that leads to the creation of bioactive and/or functional molecules.
- a) Design and Development of Chiral Brønsted Acid Catalysts Enabling Substrate Recognition and their Application to the Synthesis of Biologically Active Molecules.
- b) Development of Novel Molecular Transformations Under Brønsted Base Catalysis
- c) Development of Skeletal Rearrangements Using Transition Metal Catalysts
- d) Development of New Skeletal Construction Reactions Aimed at Creating Novel Functional π-Molecules
a) Design and Development of Chiral Brønsted Acid Catalysts Enabling Substrate Recognition and their Application to the Synthesis of Biologically Active Molecules.
a-1) Design and development of chiral Brønsted acid catalysts and catalytic reaction systems
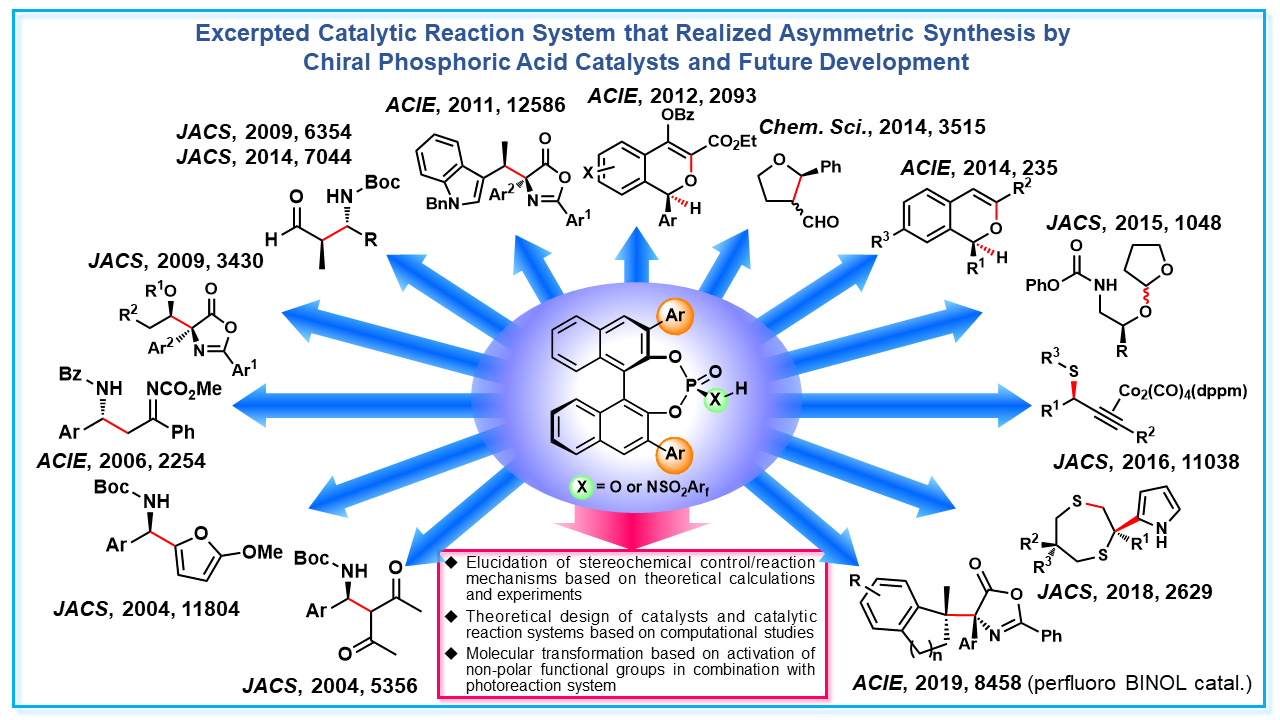
Brønsted acid is a typical catalyst that activates substrates in various organic reactions. In our laboratory, in addition to substrate activation, we have been designing and developing chiral Brønsted acid catalysts that enable substrate recognition through hydrogen bonding interaction. In particular, we have realized asymmetric synthesis, in which one enantiomer can be selectively obtained in a variety of catalytic reaction systems, using a chiral phosphoric acid developed by our laboratory. Utilizing these abundant experiences and knowledge, we are actively combining experiments and theoretical studies to clarify mechanisms of stereochemical control and are also working to design and develop catalysts and catalytic reaction systems that realize advanced stereochemical control based on theoretical calculations. In addition to the utilization of such polar functional groups as an acid catalyst, we are also investigating the activation of non-polar functional groups, that could not be used in reactions so far, by combining chiral Brønsted acid catalysts and photoreactions. By realizing molecular transformations that could not be achieved by existing methods, we aim to develop catalytic reaction systems that go beyond the framework of acid catalysts.
a-2) Development of efficient methods for synthesizing natural products and pharmaceuticals using chiral phosphoric acid catalysts
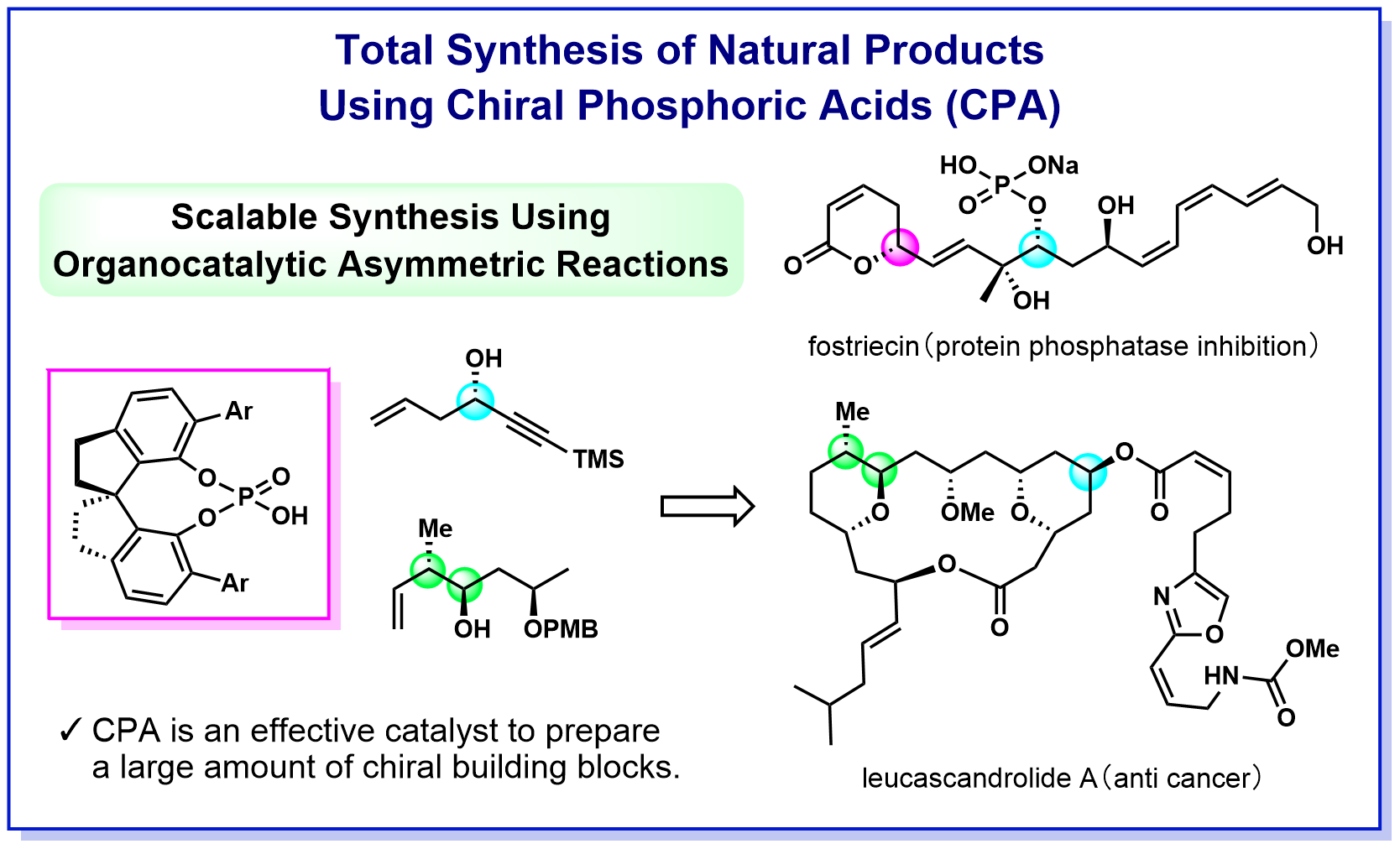
Natural organic compounds (natural products) are a treasure trove of substances with various biological activities, and mankind has used various natural products as medicines and poisons since the dawn of history. In recent years, the development of pharmaceuticals based on the structure of natural products has been actively conducted, and many compounds with activities that exceed those of natural products have been created. On the other hand, there are many natural products that can be obtained in very small quantities from nature and whose mechanism of bioactivity and medical and biological research have not progressed. Therefore, we are working to establish a process for efficient synthesis of important natural products and pharmaceuticals by utilizing the knowledge of chiral phosphoric acid catalysis that we have cultivated in our laboratory. To date, we have succeeded in synthesizing fostriecin shown below in the shortest process ever and leucascandrolide A at the highest yield ever. These synthetic routes effectively incorporate asymmetric allylation and crotylation using a chiral phosphoric acid catalyst, indicating that the catalyst developed in our laboratory is a powerful tool for total synthesis. By developing synthetic methods that achieve environmentally benign transformations and can be supplied in large quantities, we aim to contribute not only to synthetic organic chemistry but also to the field of medicinal chemistry.
b) Development of Novel Molecular Transformations Under Brønsted Base Catalysis
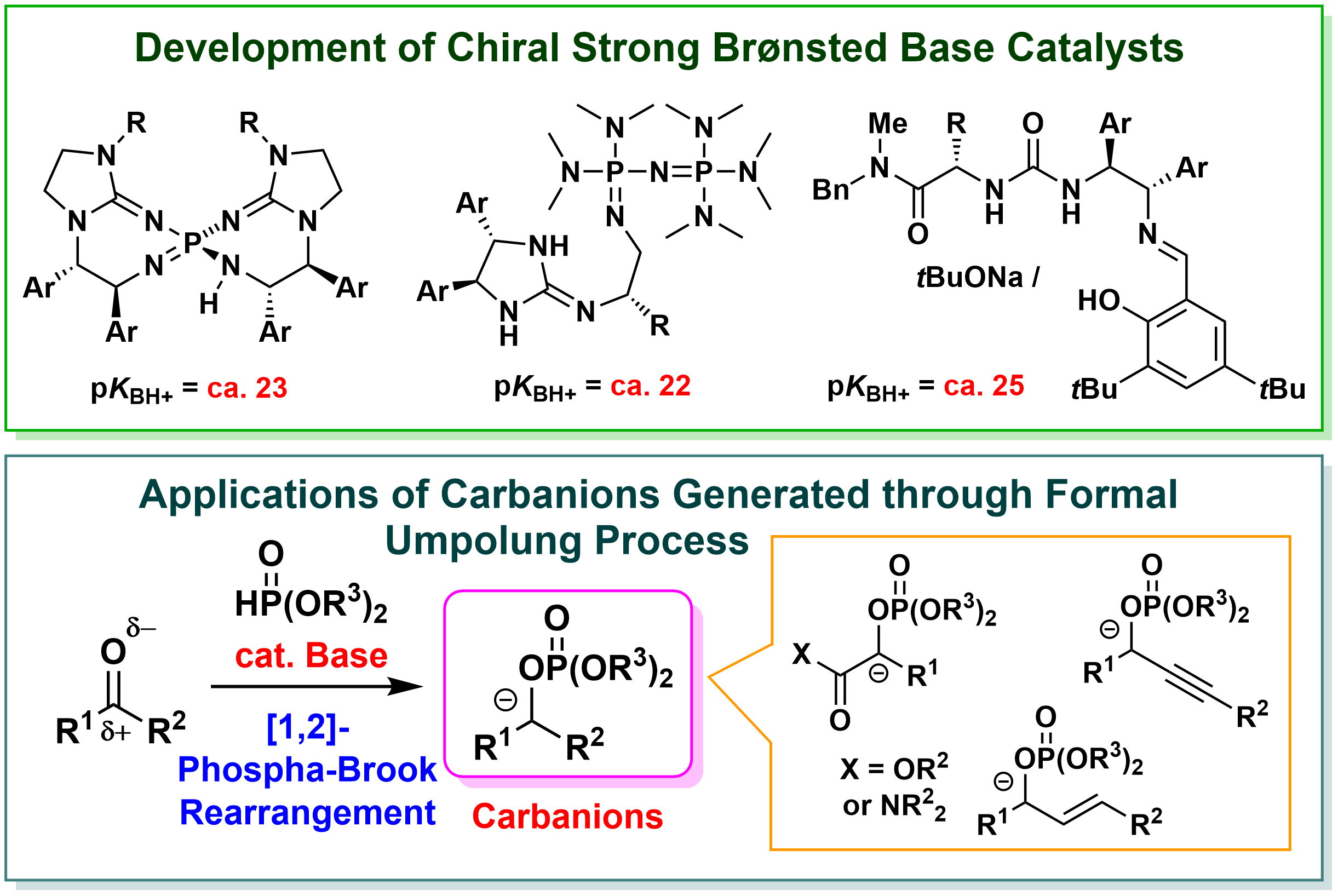
In our laboratory, we focus on Brønsted base catalysts, which are categorized as the organocatalysts (catalysts composed of small organic molecules) that are attracting attention as environmentally benign catalysts, and are working on "development of highly active chiral Brønsted base catalysts" and "design and development of advanced catalytic molecular transformations". For example, we have developed chiral Brønsted bases having much higher basicity than conventional chiral organobase catalysts, and are developing enantioselective reactions using various compounds that are difficult to apply in reactions with conventional catalysts. In addition, we have established the catalytic reaction systems that effectively utilize various anionic species and developed the methods for the synthesis of useful compounds that cannot be synthesized by conventional methods, such as heterocyclic compounds having various substituents in a positional selective manner. In this way, by realizing unprecedented highly efficient and selective molecular transformation, we aim to provide useful tools for the synthesis of various compounds that can be used in the development of new pharmaceutical and agrochemical products and organic functional materials. In addition, we aim to expand the chemical space (the ensemble of molecules) by establishing a synthetic method for unnatural amino acids, their analogs, and heterocyclic compounds, which are difficult to synthesize by conventional methods, contributing to the acceleration of life science research including drug discovery research.
c) Development of Skeletal Rearrangements Using Transition Metal Catalysts
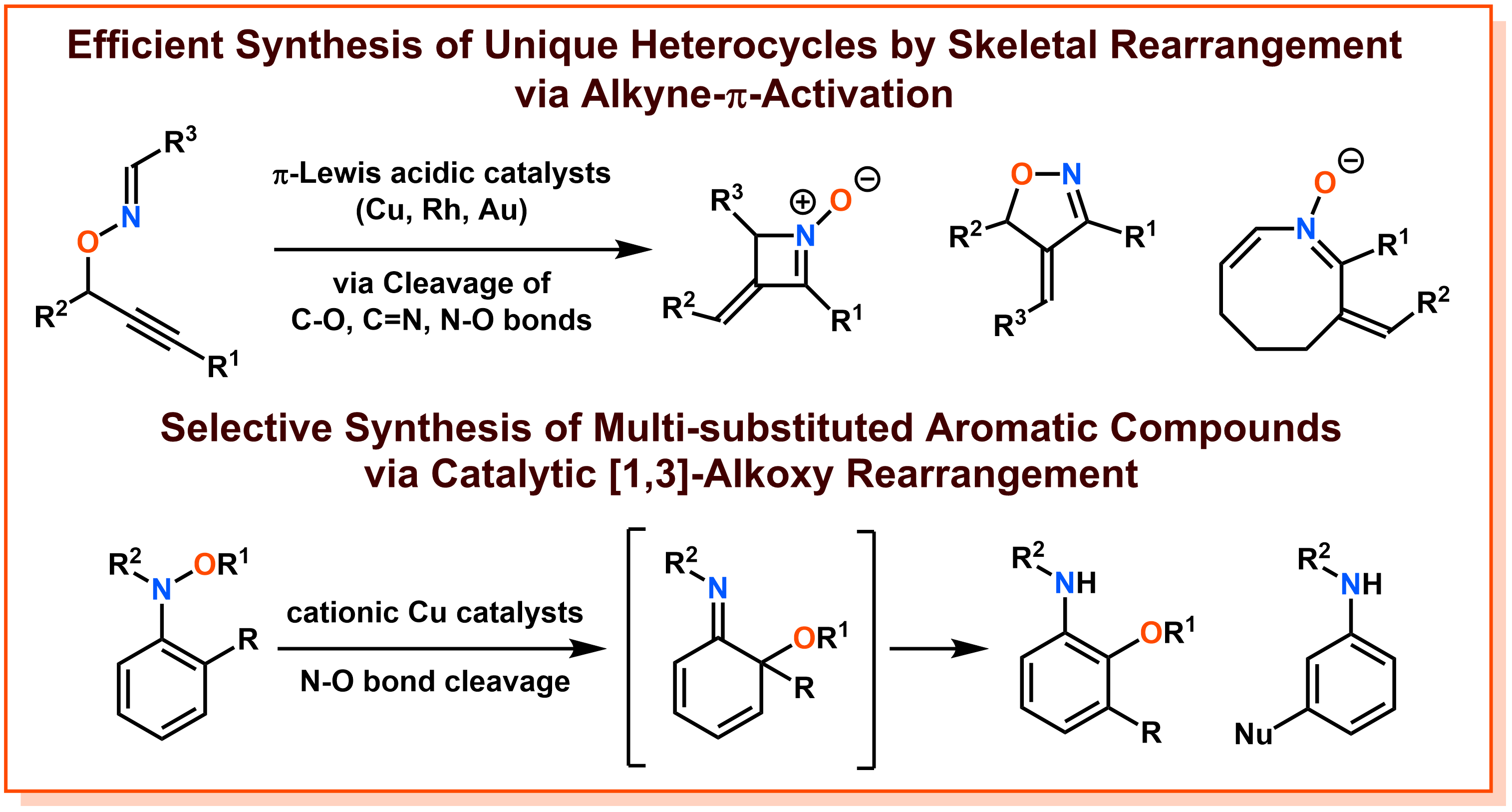
In response to the universal question of synthesizing new molecules = further expansion of chemical space, we are conducting research based on the strategy of "catalytic skeletal rearrangement". A skeletal rearrangement is a chemical reaction in which structural isomers of starting materials are synthesized through the cleavage of covalent bonds (carbon-carbon, carbon-oxygen, nitrogen-oxygen, etc.) that form the molecular backbone. In particular, by using the cleavage of weak covalent bonds as a driving force, it is expected to produce molecules that could not be synthesized by conventional methods, such as distorted molecular skeletons. Furthermore, since it is an atom-efficient process that does not lose atoms before and after the reaction, it is positioned as an environmentally benign molecular transformation required by modern chemistry. We have developed unique skeletal rearrangements through our original substrate design and careful exploration for metal catalysts including ligands, and have realized the synthesis of molecules that were difficult with conventional methods. For example, we have developed the brand-new method for synthesizing heterocyclic compounds in an unprecedented variety of heterocyclic compounds by catalytic skeletal rearrangements starting from O-propargyl oxime compounds through π-activation of their alkyne moiety. Furthermore, we discovered a cationic copper catalytic system that catalytically promotes the [1,3]-alkoxy rearrangement of N-alkoxyaniline, and developed a new method for synthesizing polysubstituted aniline derivatives. Since heterocyclic compounds and polysubstituted aromatic compounds are widely used as pharmaceuticals, we believe that the development of inventive and unique catalytic skeletal rearrangements can contribute to drug discovery. The creation of novel molecules by catalytic rearrangements has great potential, and we aim to realize new reactions and methods by further creating substrates and profoundly designing catalysts, as well as elucidating the reactivity of chemical bonds and the principle of catalysis behind them.
d) Development of New Skeletal Construction Reactions Aimed at Creating Novel Functional π-Molecules
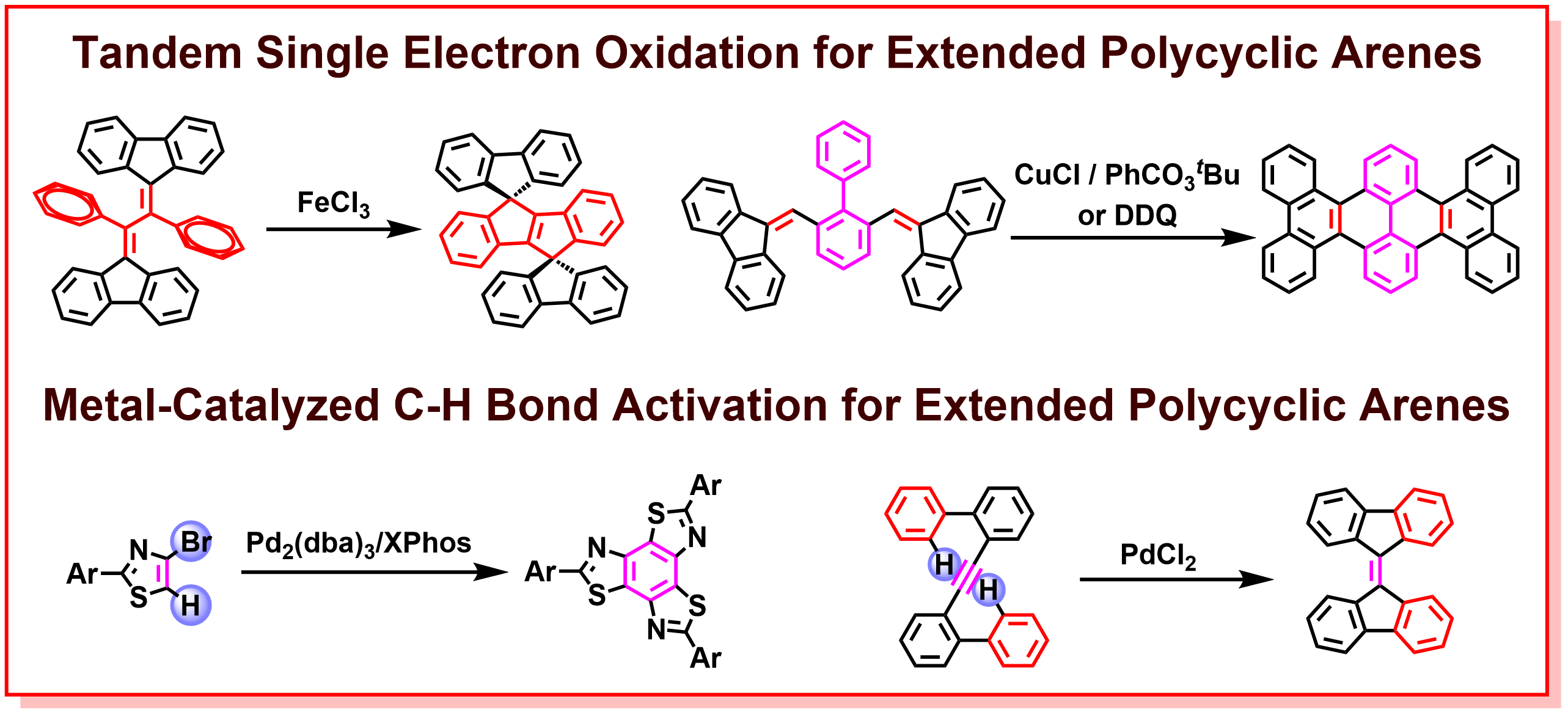
In recent years, with the rapid development of organic electronics such as organic transistors, organic electroluminescence (EL), and organic solar cells, the researches on the development of new π-based optoelectronic materials has become increasingly important. In particular, the impressive structural diversity of polycyclic arenes, such as molecular geometry, π-conjugation length, planarity, and atomic component, leads to intriguing electronic and photophysical properties, versatile intermolecular interactions, solubility, and crystallinity, which are key factors for the creation of new optoelectronic materials. Therefore, the design and synthesis of a new class of polycyclic arenes with structural diversity and excellent optoelectronic functions is an extremely important research topic in terms of diverse organic electronic applications. In our laboratory, we have been developing innovative transition-metal-catalyzed reactions, such as tandem single-electron transfer reactions and C-H bond activation reactions, for the construction of a new class of polycyclic arene skeletons with respect to the discovery of new synthetic methodologies, novel structures, and outstanding optoelectronic properties. The successful synthesis of known and unknown π-electronic molecules using newly developed synthetic methodologies is expected to provide new functional materials and design guidelines of optoelectronic molecules to the field of organic electronics.
